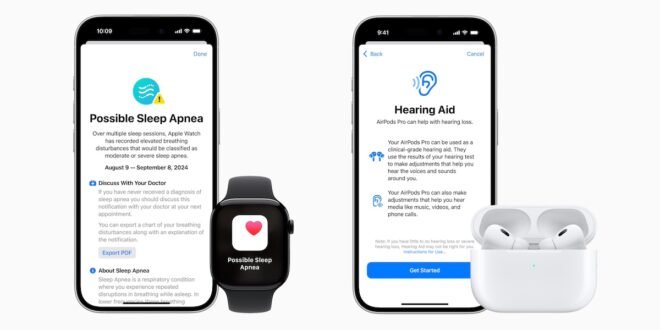
Apple has received FDA clearance for the sleep apnea feature in its newly released Watch Series 10 and over-the-counter hearing aid software embedded in its AirPods Pro headphones.
The Watch Series 10 sleep apnea feature measures whether a user has moderate to severe sleep apnea based on a new metric called Breathing Disturbances.
The device uses software algorithms and the accelerometer to analyze signals gathered over multiple sleep sessions to determine if the wearer is at risk for sleep apnea.
Users gather insights into the restlessness of their sleep within the Health App, and the Watch analyzes a wearer’s sleep data every 30 days.
If the potential for sleep apnea is detected, users receive a detailed report to show to their doctor to discuss the results.
The algorithm for the sleep apnea feature was developed using machine learning, with a dataset of sleep apnea tests, and then validated in a clinical study.
The company’s AirPods Pro headphones include clinical-grade over-the-counter hearing aids that use machine learning to automatically adjust noises to users’ hearing needs.
Wearers take a five-minute test using their iPhone and AirPods Pro to analyze their hearing health.
Users tap the iPhone screen when they hear a series of tones at different volumes and frequencies. Based on the results of that test, AirPods Pro automatically adjusts to a user’s hearing needs, serving as a clinical-grade hearing aid.
THE LARGER TREND
In 2022, the FDA issued a final rule to allow access to over-the-counter hearing aids and made related amendments to update the regulatory framework for hearing aids.
Apple announced the release of the Watch Series 10 and AirPods Pro 2 during a virtual event earlier this month, which included details on its new over-the-counter sleep apnea and hearing aid technologies.
Other companies in the at-home sleep apnea testing space include U.K.-based wearable medical device company Acurable, which offers its sleep-testing device AcuPebble SA100 that enables users to detect and monitor obstructive sleep apnea.
Belgian sleep-focused digital health startup Sunrise received FDA 510(k) clearance last year for its home sleep apnea test that is placed on a user’s chin at night.
In the over-the-counter hearing aid space, digital health company Soundwave Hearing announced it received FDA 510(k) clearance last year for its Sontro Self-Fitting OTC Hearing Aids. Its offering blends mobile technology and AI calibration to self-fit and amplify sound for individuals with mild to moderate hearing impairment.
Earlier this month, hearing aid manufacturer Signia revealed its new product, Signia Active Pro Integrated Xperience (IX) earbuds, which also qualify as prescription-grade hearing aids.